
byCollegedunia Team Content Curator
SAT chemistry is among the 20 broad subjects offered in SAT subject test. SAT chemistry subject test comprises 85 MCQs with a duration of 60 minutes. Candidates planning to pursue engineering or science-related undergraduate programs mainly take SAT chemistry. SAT chemistry subject test examines candidate’s ability to implement principles and solve problems. SAT chemistry is scored on a range of 200 to 800. The best book for SAT subject test chemistry is both Barron’s and the Official Subject Test Guide.
SAT Chemistry Subject Test Syllabus
SAT chemistry subject test syllabus examines the fundamental concepts and skills, application, and synthesis of knowledge. Candidates need to show their ability to arrange and derive results from observations and experimentations. Candidates can check their knowledge level by taking a free SAT chemistry practice test. SAT chemistry subject test doesn’t allow a calculator. SAT exam dates of chemistry exam are available in the months of August, October, November, December, May, and June. A periodic table is also provided in SAT chemistry subject test. Below is the graph showing the topics and their weightage in SAT Chemistry exam:
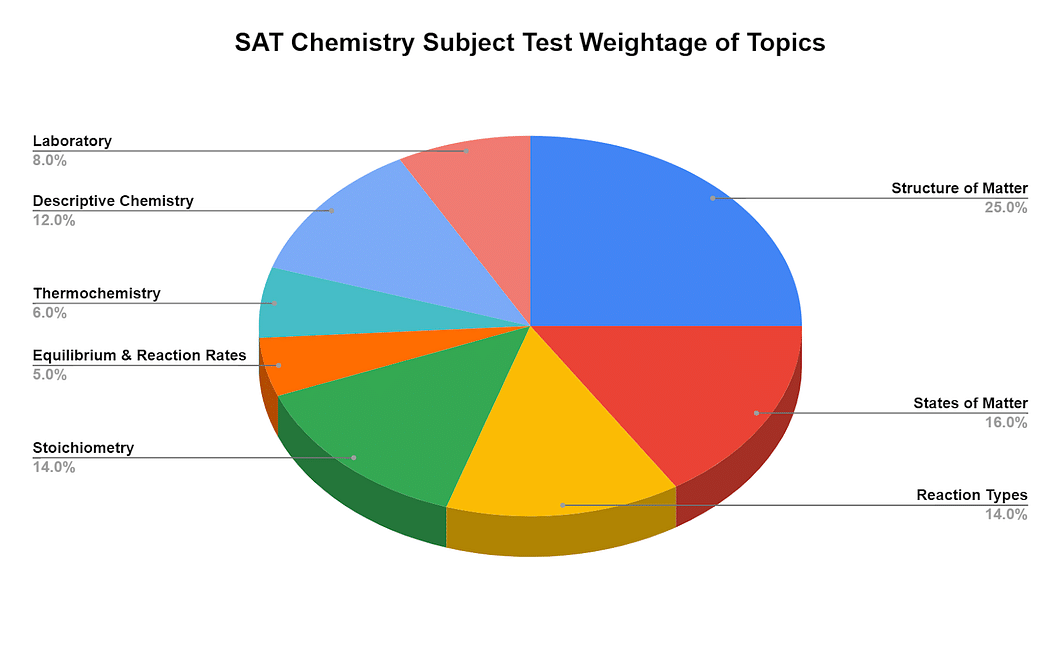
Candidates can consult from SAT chemistry PDF that contains a detailed description of concepts. The topics contain many sub-topics that candidates need to learn. Below is the detailed SAT chemistry subject test syllabus with the breakdown of the topics:
| SAT Chemistry Subject Test Topics | Breakdown of Areas | Sub-areas |
|---|---|---|
| Structure of matter | Atomic Structure | Experimental testimonies of atomic structure, quantum numbers, energy levels, electron configurations, periodic trends |
| Molecular Structure | Lewis structures, 3-dimensional molecular shapes, polarity | |
| Bonding | Ionic, covalent, metallic bonds, the connection of bonding to properties & structures, intermolecular forces, | |
| States of Matter | Gases | Kinetic molecular theory, gas law connection, molar volumes, density, stoichiometry |
| Liquids and solids | Intermolecular forces in solids/liquids, phase changes & diagrams | |
| Solutions | Molarity & percent by mass concentrations, solution preparation & stoichiometry, factors affecting the solubility of gases/liquids/solids, colligative properties | |
| Reaction Types | Acids and bases | Bronsted – Lawry theory, strong/weak acids, pH, titrations, indicators |
| Oxidation and reduction | Identification of oxidation-reaction reactions, combustion, oxidation count, activity series | |
| Precipitation | Fundamental solubility rules | |
| Stoichiometry | Mole concept | Molar mass, Avogadro’s number, empirical/molecular formulas |
| Chemical Equations | Balancing, calculations, percent yield, restricting reactants | |
| Equilibrium & Reaction Rates | Equilibrium systems | LeChâtelier's principle, equilibrium constants & expressions |
| Rates of Reaction | Reaction rates, potential energy diagrams, activation energies | |
| Thermochemistry | Conservation of energy, calorimetry, enthalpy, heating/cooling curves, entropy | - |
| Descriptive Chemistry | Fundamental elements, ions, compounds, periodic trends, the reactivity of elements | - |
| Laboratory | Awareness of lab equipment, measurements, procedures, observation, security, calculations, data analysis, and more | - |
Is the SAT Chemistry Subject Test Hard?
SAT chemistry subject test is not hard but requires rigorous practice and dedication. SAT chemistry formula sheet along with SAT chemistry tips are helpful preparation materials. SAT chemistry subject test is mostly filled with theoretical concepts and less numerical ones and also the SAT 2 chemistry curve. Candidates can start by practicing from SAT chemistry practice test PDF, and consulting a SAT 2 chemistry book.
SAT Chemistry Subject Test: Question Types
The Collegeboard SAT chemistry subject test comprises three categories of questions. For cracking the SAT subject test in Chemistry, candidates need to know each question type:
- Classification questions: Here, candidates will find a list of choices that can be applied to a group of numerous questions. Candidates need to select one answer that suffices all the questions or statements. Then write the answer in the bubble in the answer sheet. The choice can be used once, more than once, or not at all in individual sets.
- Relationship analysis questions: There are two statements in I and II connected by ‘because’. Candidates need to determine if both of them are true or false. Then eventually write the same in the bubble in the answer sheet. The bubble ‘CE’ should be marked only if statement II is an accurate representation of statement I. Candidates need to practice this question type from SAT chemistry online practice test.
- 5 - Choice completion questions: These questions are either statements or incomplete statements whose accurate answer has to be selected from the multiple-choice answers provided. Candidates can practice from SAT 2 chemistry book to know more about this question type.
SAT Chemistry Subject Test: Score
SAT chemistry is marked on an SAT score scale of 200 - 800. SAT subject test scores are marked either 30 - 40 points above or below the original score. This happens because the scores cannot determine the actual ability of the candidates based on a single paper. Furthermore, the candidate’s SAT total score can differ based on the test days. There are many SAT chemistry online practice test that helps candidates by providing scores. The College Board notifies candidates with a link to access their SAT subject test score reports once it is out.
SAT Chemistry Subject Test: Percentile Rankings
SAT subject tests have a percentile score which marks the ranking of the candidate compared to the other candidates taking the exam. If a candidate’s percentile rank for 500 is 47, this states that the candidate has scored better than 47% of the remaining candidates.
| SAT Chemistry Subject Test Scores | Percentile Ranks |
|---|---|
| 800 | 90 |
| 750 | 71 |
| 700 | 53 |
| 650 | 38 |
| 600 | 24 |
| 550 | 14 |
| 500 | 8 |
| 450 | 3 |
| 400 | 1 |
| 350 | 1- |
SAT Chemistry Subject Test: Evaluation Method
The SAT chemistry subject test score is calculated in a rigid manner following certain steps. The answers are marked in circles. The raw score is the total of correct answers on SAT chemistry answer sheet. From that raw score, a fraction of the number will be deducted in case of inaccuracy.
- 1 point for every accurate answer;
- Deduction fractions:
- ¼ is deducted in case of 5 MCQs;
- ⅓ is deducted in case of 4 MCQs;
- ½ is deducted in case of 3 MCQs
- Unattended questions will cost no marks
- In case the actual result appears in a fraction, the score is rounded to the nearest whole number.
What is a Good SAT Chemistry Score?
Generally, most of the top universities require a high SAT score of around 700 for admission. Similarly, for SAT subject tests, top universities like MIT required above 700. Though there are candidates securing admission even with lower scores it is generally advised to aim for higher scores. Candidates should rigorously follow SAT chemistry book which ranks the highest and get themselves prepared. Every single score is necessary to bag a seat in the top-ranking universities, and therefore, candidates should go through all the SAT 2 chemistry book.
Best Book for SAT Subject Test Chemistry
Candidates need to prepare from SAT chemistry book to score high. SAT 2 chemistry book contains numerous practice tests, solutions, explanations of complex topics, sample questions, and more. We have listed a few of the best book for SAT subject test chemistry here:
- Barron’s SAT Subject Test Chemistry 11th Edition – Check PDF
- McGraw Hill’s SAT Subject Test Chemistry – Check PDF
- Cracking the SAT Subject Test in Chemistry – Check PDF
- Kaplan SAT Chemistry Subject Test
These are a few of the top SAT chemistry PDFs that the candidates can choose from depending on their specifications.
*The article might have information for the previous academic years, which will be updated soon subject to the notification issued by the University/College.






Comments